Research
Research topics
Experimental equipments
Modelling activity
Research Projects
Seminars
Awards
Experimental Equipment
The diffusion in polymers lab at the University of Bologna is one of the most complete ones for the characterization of the mass transport and sorption properties in polymers and solids.
The apparatuses work in a wide range of operative conditions (pressure, temperature, composition, humidity), and with different types of materials (powders, pellets, barrier materials, permeable membranes).
The permeability, diffusivity, solubility, relaxation, dilation, and stress phenomena of polymers in presence of gases, vapors and liquids can be studied.
Experiments can be carried on with dry gases, humid gases or mixed gases.
Some permeation and sorption experiments are carried on according to the ASTM D 1434.
Overview
Fluid Sorption
Fluid Permeation
Dilatometry & Rheology
Film Fabrication
General Analytical Equipment
Videos
Fluid Sorption and Diffusion in Solids
These instruments allow to determine the fluid solubility inside a solid material and, if the sample has a regular geometry, also the fluid diffusivity. They rely on gravimetric, manometric, piezometric, deformation, IR or magnetic measurements. Depending on the maximum pressure applicable in the system they are more suitable for characterization of the transport of vapors (low pressures) or gases (high pressures) in the solid samples.
Electrobalance Cahn (vapor sorption)
Quartz Spring Balance (vapor sorption)
Quartz Crystal Microbalance (QCM) (gas and vapor sorption)
Rubotherm Magnetic Balance (gas sorption)
Pressure Decay for vapor sorption
Pressure Decay for gas sorption
Pressure decay for mixed gas sorption
FTIR-ATR Spectrometer (mixed vapor and liquid sorption and
diffusion)
Bending cantilever
Fluid permeation in Solids
Closed Volume Apparatus for Ultrabarrier Polymers
(gas and vapor permeation)
Closed Volume Apparatus for Membranes (gas
and vapor permeation)
Closed Volume Apparatus with Controlled Humidity
(humid gas permeation)
Mixed Gas Apparatus with Controlled Humidity (humid
mixed gas permeation)
Mixed gas high temperature Apparatus (mixed gas permeation, high T)
Dilatometry and rheology of polymers and solutions
Micrometer for polymer swelling
Film fabrication
Spin coater
Roll coater
General Analytical Equipment
Micro Gas Chromatograph (H2, He, N2, O2, CO2, CH4,
...-n-C4)
Mass spectrometer
HPLC
UV Spectrometer
Electrobalance Cahn
This apparatus is built to perform vapor sorption and diffusion experiments
in polymers, from penetrants in the vapor phase. To this aim, the sensible
element, a Cahn electrobalance D-101, is coupled with a vapor-generating
section and a vacuum pump to evacuate the system. The sample is hung at
the balance left arm, while an inert tare is suspended at the opposite
arm. This apparatus can measure differences between sample weights with
the sensitivity of 10-6 times their absolute value. The vapor generator
is realized by a vacuum-proof glass vessel, partially filled with the
penetrant in the liquid phase and immersed in a thermostatic bath. A vapor
reservoir of 2 L capacity is inserted between the balance and the vapor
generator. The electrobalance, the reservoir are inclosed in a container
in which the temperature is controlled to the set-point value within 0.1°C.
An electrovalve is inserted between the reservoir and the balance in order
to suddendly realize changes in pressure which can be very rapid during
sorption experiments. The maximum pressure in the electrobalance
is 1 atmosphere, while temperatures range from the dew temperature of
the penetrant to 125°C. The sample weight is recorded and plotted
by a computer. This apparatus was used to perform sorption and diffusion
measurements of organic vapors (alkanes and alcohols) into high free volume
glassy polymers such as poly[1-(trimethylsilyl)-1-propine] (PTMSP), poly[terbutylacetylene](PTBA),
Poly(2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole -co-tetrafluoroethylene)(amorphous
TEFLON). Also rubbery polymers, such as poly[dimethylsyloxane], perfluorinated
elastomers and polyetherurethane, were characterized.
Related papers:
F. Doghieri, D. Biavati, and G. C. Sarti, "Solubility and Diffusivity
of Ethanol in PTMSP: Effects of Activity and of Polymer Aging", Ind.
Eng. Chem. Res. 1996, 35, 2420-2430.
This apparatus is based on a spring of quartz on which the polymeric sample is supended and immersed in the pentrant phase. The elongation of the spring is monitored with a camera, directly connected to a computer for data registration, and gives a measure of the weight change of the polymer due to the sorption of gas. The digital camera also allows for measuring the superficial dilation of the sample due to sorption.
Related papers:
E. Piccinini, M. Giacinti Baschetti., G.C. Sarti, "Use of an automated
spring balance for the simultaneous
measurement of sorption and swelling in polymeric films", Journal of
Membrane Science 234 (2004) 95–100.


Quartz Crystal Microbalance QCM
A quartz crystal microbalance (QCM) has been setup for the determination
of vapor sorption and diffusion in polymeric films (for a description of
this technique applied to the study of gas sorption in polymers, see for
instance C. Zhang, B.P. Cappleman, M. Defibaugh-Chavez, and D.H. Weinkauf,
J. Polym. Sci. Part B:Polym. Phys.2003, 41, 2109-2118.). The data obtained
with this technique match well with data from other techniques, validating
Sauerbrey's equation, (G. Sauerbrey, Z. Phys. 1959, 155, 206-222) that relates
the mass uptake to the measured frequency shift and to the polymers and
quartz crystal parameters, as a suitable method to analyse the data also
for the glassy polymers :
Sauerbrey's equation
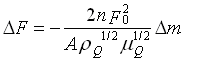
DF : frequency shift (Hz)
Dm : mass uptake (g)
n : order of harmonic
F0 : base frequency of the crystal (Hz)
A : area of the electrode (cm2)
rQ : density of the crystal (g/cm3)
mQ : shear modulus of the crystal
Related papers:
Y. Yamamoto, M. C. Ferrari, M. Giacinti Baschetti, M. G. De Angelis, G. C. Sarti
"A quartz crystal microbalance study of water vapor sorption in a short side-chain PFSI membrane"
Desalination, 200, 1-3, 2006, 636-638.
Temperature up to 500 °C
Pressure up to 150 Bar
Precision 1 microgram; Reproducibility 2 micrograms
This instrument can measure with great accuracy the equilibrium and transient sorption and adosorption of gases (even aggressive ones) in polymer films, powders, metal etc. The system can work at high temperatures and pressures.
View a presentation of this instrument
In the pressure decay technique, a known amount of vapor is fed into the sample chamber and the mass uptake is evaluated by measuring the pressure decrease in the gaseous phase; the equilibrium solubility is equal to the final, asymptotic value of the mass uptake. Subsequent sorption tests are performed by increasing the external pressure in a stepwise manner. For vapor penetrants, the measurements are conducted at pressures below 1 bar; pressure is measured with an absolute capacitance manometer (f.s. value 1000 or 100 mbar, accuracy 0.15% of the reading). The system is placed in an air-thermostatted chamber where the temperature is fixed to within 0.1 C.
Related papers:FERRARI M.C., GALIZIA M., DE ANGELIS M.G., SARTI G.C. (2010). Gas and Vapor Transport in Mixed Matrix Membranes Based on Amorphous Teflon AF1600 and AF2400 and Fumed Silica. INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, vol. 49, p. 11920-11935.
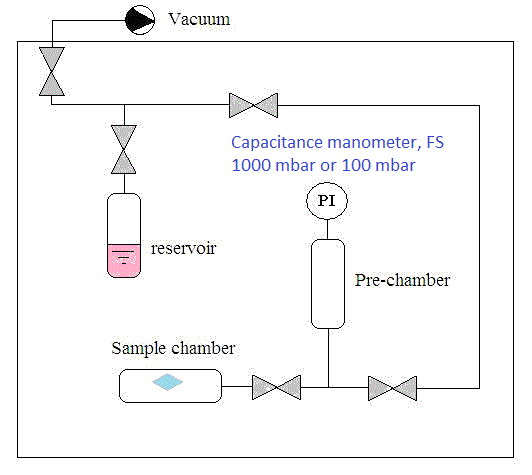 |
| Pressure decay for vapors(Paper) |
For gas sorption experiments, the pressure is monitored by two manometers (Sensotec Super TJE), the first one having a full scale of 200 absolute psi, the second one of 500 absolute psi. Both manometers have an accuracy equal to 0.05 % of the full scale value and they can be connected separately to the experimental chamber in different pressure intervals within the same isothermal experiment, in order to maintain a good level of sensitivity at any pressure.
Related papers:FERRARI M.C., GALIZIA M., DE ANGELIS M.G., SARTI G.C. (2010). Gas and Vapor Transport in Mixed Matrix Membranes Based on Amorphous Teflon AF1600 and AF2400 and Fumed Silica. INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, vol. 49, p. 11920-11935.
 |
| Pressure decay for gases (24) |
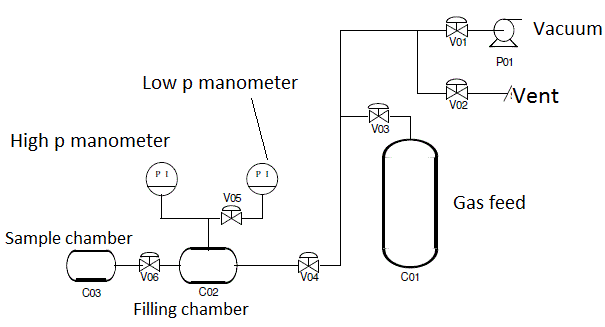
Pressure decay for Mixed Gas Sorption
It is a pressure decay equipment where the total pressure decrease versus time is monitored, as well as the equilbrium composition of the head space of the sample compartment, by means of a micro GC. This procedure allows to evaluate the binary gas solubillity in polymers and solids up to 35 bar of total pressure. The apparatus is thermostatted in a water bath. Sensors: Honeywell STJE absolute pressure gauges of the full-scales of 100 psia, 200 psia and 500 psia.Gas chromatograph Varian CP-4900 Micro-GC equipped with the capillary column (Varian PoraPLOT U, length 10 m, inner diameter 0.32 mm) and with the thermal conductivity detector. The main part of the apparatus, except the pressure gauges, is fully submerged in a water bath. A oil-sealed rotary vacuum pump Edwards RV3 was used. The apparatus can be used with binary gas mixtures, and it detects CH4, CO2, N2, O2, hydrocarbons up to n-C4.
Related papers:Ondrej Vopicka, Maria Grazia De Angelis, Giulio Cesare Sarti, Mixed gas sorption in glassy polymeric membranes: I. CO2/CH4 and n-C4/CH4 mixtures sorption in poly(1-trimethylsilyl-1-propyne) (PTMSP), J Membrane Sci 2014, 449, pp. 97 - 108
 |
 |
| Mixed gas sorption apparatus picture | Mixed gas sorption apparatus scheme |
The FTIR-spectroscopy (Infrared Spectroscopy with Fourier Transform) is based on the property, characteristic of any material, of absorbing light at a determined wavelength. For mass transport applications, in particular, the ATR (Attenued Total Reflection) configuration is employed: this technique allows for the analysis "in situ" of diffusive transport of gases and liquids in polymeric films. The most appealing features of this tecnique are: - Possibility of studying the diffusion of multicomponent systems, in particular of directly measuring the diffusion coefficient of each component of a mixture diffusing into a polymeric film - Ability of recognizing any interaction between the penetrant and the polymer and of estimating its influence on the diffusion process - Possibility to detect and to qualitatively analyze the swelling induced by the penetrant in the polymer during sorption The analytical technique is based on the observation that, in each phenomenon of total reflection there is a penetration of the electromagnetic field in the less dense phase, the so-called "evanescent wave". This wave is selectively absorbed by the film, which leaves its characteristic track in the light beam. The spectrometer registers this track and yields an interferogram (intensity as a function of time), which is then recomputed through a Fourier transform, so to give an absorption spectrum (absorbance as a function of wavelength), where one can determine the peaks of the polymer and of the penetrants. Study of these peaks easily leads to the value of concentration of the penetrant in the polymer and of the diffusion coefficient.
Related papers:
Giacinti Baschetti, M.; Piccinini, E.; Barbari, T. A.; Sarti, G. C.; " Quantitative Analysis of Polymer Dilation during Sorption Using FTIR-ATR Spectroscopy ", Macromolecules; (2003); 36(25); 9574-9584.
Yossef A. Elabd, Marco Giacinti Baschetti, Timothy A. Barbari, "Time-resolved Fourier transform infrared/attenuated total reflection spectroscopy for the measurement of molecular diffusion in polymers" Journal of Polymer Science Part B: Polymer Physics Volume 41, Issue 22, Date: 15 November 2003, Pages: 2794-2807.
M.C. FERRARI, J. CATALANO, M. GIACINTI BASCHETTI, M. G. DE ANGELIS, G. C. SARTI (2012). FTIR-ATR Study of Water Distribution in a Short-Side-Chain PFSI Membrane. MACROMOLECULES, vol. 45, 1901-1912


A technique for the characterization of relevant mechanical properties (swelling and stress build-up) playing a role during sorption in glassy polymers has been set up and different macroscopic models to analyse the data have been developed . Indeed, a bending beam technique has been developed suitable to measure during sorption time the deflection of the free end of a cantilever, clamped at one end, coated with a polymeric film which undergoes sorption.[H.M. Tong, K.L. Saenger, in H.M. Tong, L.T. Nguyen, New Characterization Techniques for Thin Polymer Films, Wiley Interscience, New York, NY, 1990] The free swelling of the polymer during sorption of a vapor is inhibited by the substrate: this induces a curvature in the beam and also a state of compression in the film; the stress profile in the film can be calculated; the polymer swelling can also be reliably measured if one uses a cylinder as support.
Related papers: M. Ferrari, E. Piccinini, M. Giacinti Baschetti, F. Doghieri, G. Sarti, Solvent induced stresses during sorption in glassy polycarbonate: experimental analysis and model simulation for a novel bending cantilever apparatus, INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 2008, 47, pp. 1071 - 1080.

Closed Volume Apparatus for Ultrabarrier Polymers
This apparatus was conceived for the characterization of gas permeability in barrier polymeric films according to ASTM D1434 It is a fixed volume, variable pressure apparatus. It has two membrane cells, both of the type Millipore XX45 047 00. The downstream volume of the cell is in both cases equal to about 30 cm3, so to increase the resolution of the system, and two capacitance manometers (Edwards Barocel and MKS Baratron), Full Scale=10 mbar, are used to detect the downstream pressure. Fittings are all Cajon Vacuseal, 1/4", and the whole system is air thermostatted (+5-+80 C). The upstream pressure is measured with an absolute manometer (Druck, PCDR 4070, FS 10 bar). Gas permeability downto 10^-3 Barrer can be explored with this apparatus. (OTR: 10^-1 g/cm2 day bar for a 40 micrometer thick film) as well as the gas diffusivity with the time lag method.
Related papers:
G. Malucelli, E. Amerio, M. Minelli, M.G. De Angelis "Epoxy-siloxane hybrid coatings by a dual-curing process", Advances in Polymer Technology, Vol. 28, No. 2, 77–85 (2009).
M. Minelli, M.G. De Angelis, F. Doghieri, M. Rocchetti, Marco, A. Montenero "Barrier properties of organic-inorganic hybrid coatings based on polyvinyl alcohol with improved water resistance", Polymer Engineering and Science, 2010,50,144-153,.
J. Catalano, M. Giacinti Baschetti, M. G. De Angelis, G. C. Sarti, A. Sanguineti, P. Fossati "Gas and water vapor permeation in a short-side-chain PFSI membrane" Desalination 240 (2009), 341-346.
M. Minelli, M.G. De Angelis, F. Doghieri, M. Marini, M. Toselli, F. Pilati
"Oxygen permeability of novel organic-inorganic coatings: I. Effects of organic-inorganic ratio and molecular weight of the organic component", European Polymer Journal, 44, 2581-2588 (2008).
Closed Volume Apparatus for Membranes
This apparatus was conceived for the characterization of gas permeability in permeable membranes according to ASTM D1434 like the one described above. It is a fixed volume, variable pressure apparatus. It has one membrane cell, both of the type Millipore XX45 047 00 or similar. The downstream volume of the cell is in both cases equal to around 25 cm3 and one capacitance manometers (Edwards Barocel),Full Scale=100 or 1000 mbar, are used to detect the downstream pressure. Fittings are all Cajon Vacuseal, 1/4", and the whole system is air thermostatted (+5-+80 C). The upstream pressure is measured with an absolute manometer (Druck, PCDR 4070, FS 10 bar). Gas diffusivity with the time lag method can also be detected with the present apparatus.
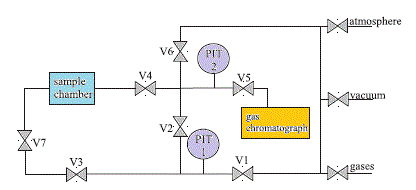
Closed Volume Apparatus with controlled humidity
Watch a VIDEO ITALIAN The apparatus for the determination of humid gas permeability was adapted from a pure gas, fixed volume permeometer. In particular, a retentate stream was added, as well as a humidity controller system on the upstream side of the membrane. The scheme of the apparatus is shown in Figure 2. The downstream volume is equal to 35 cm3, a high pressure manometer is placed in the upstream side (PI01, Druck f.s. 6 bara) as well as an absolute manometer (PI03: MKS Baratron f.s. 100 mbar) and a differential manometer in the downstream side (PI04: Differential Druck manometer, f.s. 10 mbar). For humidity control, a humidity sensor (RH 01: Vaisala RH: 0 -100 %; T : - 40/+80 C) is used, and a thermocouple (TI01) is placed in the bubbling vessel S02 (7 dm3). S01 is a vessel for pure gases that is used for pure gas permeation experiments, and S03 is the water reservoir (2 dm3) that is used for the water equilibration stage and can be used for pure water vapour permeation experiments. The experiment is carried on by first putting the membrane in contact, on both sides, with water vapor at fixed activity, fed from vessel S03, and let the system reach equilibration. The same activity value will be kept constant throughout the permeation experiment. The end of this saturation stage is reached when a uniform water concentration profile is attained, that is indicated by a constant pressure value on both sides of the membrane: in the conditions inspected it normally lasts a few hours. After the saturation, valve V06 is closed, in order to isolate the upstream and downstream volumes of the apparatus, and the upstream conditions are varied by feeding a gas stream with a fixed humidity value. The upstream pressure is set by tuning the gas partial pressure, read through PI04, through the pressure reducer in the gas feed. The stream humidity is adjusted by tuning the valve on the bubbling vessel (V05) and on the gas bypass line (V04), to a value equal to the activity value kept during the previous hydration stage. In this way, a driving force for gas permeation is established, while the driving force for the water flux is equal to zero as in the previous step: moreover the presence of a retentate flux allows to avoid any possible concentration polarization effect. The pressure increase in the downstream volume is due to the permeation of pure gas, and allows for a simple quantification of gas permeability under humid conditions with the same equations used for pure gas permeation. Moreover, in this device the presence of a purge stream avoids any possible concentration polarization effect. For experiments below 40 C, the water vapour pressure is lower than 100 mbar that is the full scale value of manometer PI03, which is used to measure pressure both in the saturation stage and in the permeation run. At higher temperatures, one can proceed by simply replacing the manometer with a higher full scale value one, or, in order not to loose the sensitivity on pressure, in a different way, that is depicted in Figure 3. In the downstream side we placed a differential manometer PI04 with a low FS value (10 mbar) and high resolution. The valve V07 is kept open during the saturation stage, so that the p value read by the manometer is zero. At the beginning of the permeation run, the valve V07 is closed, so that the volume comprised between V07 and the manometer PI03 remains at the initial pressure value of the saturation stage, while the other end of the differential manometer reads the permeate pressure that increases with time due to gas permeation. In this way, the value of P(t) read is directly related to the mass of gas permeated, from which the permeability can be easily evaluated. This system allows to work at pressure higher than 100 mbar with good resolution.
Related papers:
CATALANO J., MYEZWA T., DE ANGELIS M.G., GIACINTI BASCHETTI M., SARTI G.C. (2012). The effect of relative humidity on the gas permeability and swelling in PFSI membranes. INTERNATIONAL JOURNAL OF HYDROGEN ENERGY, vol. 37, p. 6308-6316.
M. Minelli, M. Giacinti Baschetti, F. Doghieri, M. Ankerfors, T. Lindstrom, I. Siroc, D. Plackett Investigation of mass transport properties of microfibrillated cellulose (MFC) films Journal of Membrane Science 358 (2010) 67-75.
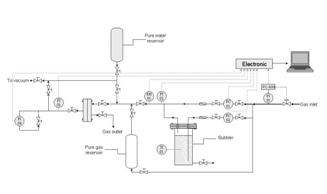
Mixed Gas Apparatus with Controlled Humidity
Watch a VIDEO ITALIAN A mixed gas permeation apparatus is available to perform mixed gas measurements in membranes, where the feed flow is regulated with mass flow controllers, the temperature is controlled with a water bath and the permeate flow is measured with flow meters, while the permeate is sampled and analyzed at fixed time intervals with a micro gas chromatograph. A scheme of this apparatus is shown in Figure below.

Mixed Gas High Temperature Apparatus
A custom-built stainless steel tube-in-shell membrane module is used to test key transport properties of palladium coated membranes for hydrogen pourification from steam reforming streams. VCR fittings sealed the membranes to an internal holder, and a copper gasket formed the seal at the shell flange. All joints and seals are tested at room temperature under a differential pressure of 3 bar to ensure absence of detectable leaks. Feed gases flow into the pressurized shell side of the module, and permeate across the membrane or leave through the retentate exit. Bottled feed gases of H2, N2 and CO, available at 10 bar, are metered using three mass flow controllers [Bronkhorst High-Tech El-Flow] to obtain the desired flowrate and composition. Permeate and retentate/feed pressures are maintained through back pressure regulators [Bronkhorst High-Tech El-Press], while flow rates were metered with mass flow meters [Bronkhorst High-Tech El- Flow]. All controllers and meters are monitored, recorded and adjusted through an internal PC server, which communicates to the controllers and meters through a Bronkhorst dynamic data exchange (DDE). The server relayed data to a set of PC software interfaces [Labview code & Bronkhorst Flowview]. An oven [Carbolite GHL 12/300] is used to house the tube-in-shell module, and maintain an isothermal environment over the entire membrane module. A thermocouple adjacent to the membrane coating allows a manual optimization of the oven set point. Feed gases are preheated to the module temperature by first passing through a preheater tube inside the oven. A Varian Micro Gas Chromatographer unit (CP-4900 Molesieve 5 Angstrom with Argon as carrier gas) analyses the actual compositions of feed, permeate and retentate streams, which were injected into the unit during testing. Connection to a PC allowed for data acquisition and control through a software interface [Varian Galaxie Workstation]. Pressure on the permeate side was maintained at atmospheric conditions, and no sweep gaswas used on the permeate side during any experiments. The tests are conducted inside a hood, with a Bunsen burner to safely handle the flammable gases exiting the module. Additional safetymeasureswere setup through an extractor fan located in the cupboard, and three concentration activated feed shut-off valves for CO, H2 and CH4 detection, respectively.
Related Papers J. Catalano, M. Giacinti Baschetti, G.C. Sarti "Influence of the gas phase resistance on hydrogen flux through thin palladium-silver membranes Journal of Membrane Science, v 339, n 1-2, p 57-67, September 1, 2009
D. Pizzi, R. Worth, M. Giacinti Baschetti, G.C. Sarti, K. Noda Hydrogen permeability of 2.5 micron palladium-silver membranes deposited on ceramic supports Journal of Membrane Science, v 325, n 1, p 446-453, November 15, 2008.
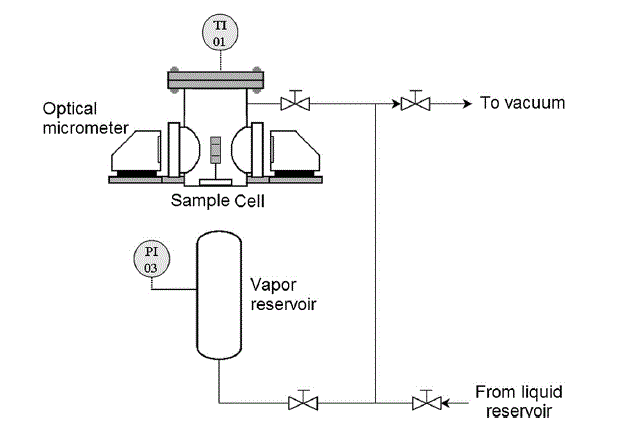
Micrometer for polymer swelling
The system is composed of a special sample compartment, formed by a stainless steel cell endowed with two borosilicate glass windows in the opposite sides, to allow optical access for the measurements. The cell is connected to a reservoir containing the penetrant vapor and the pressure transducer, and to a second flask for the storage of liquid penetrant. A vacuum system, with a liquid nitrogen trap and a vacuum pump, is used to evacuate the apparatus before and after each experiment. The sensing element is a high accuracy digital micrometer (Keyence LS-7030-M) endowed with a high speed linear CCD sensor that ensures an accuracy of 1 Ám and a reproducibility to within 0.15 micrometer in the measurements. The measuring head features a gallium nitride (GaN) green LED whose light is converted into a parallel beam by a system of lenses, and it is directed on the cantilever through the borosilicate windows. The LED beam reaches then a telecentric optical system that uses only parallel light to form the image on the linear CCD sensor. The latter prevents fluctuations in lens magnification due to a change in target position and ensures an accurate measurement of the signal formed by bright and dark areas that correspond to the sample shade on the CCD. The measuring system is also provided with small CMOS image sensor for control purposes. The software used for signal elaboration is able to measure simultaneously two different distances between points characterized by a brightness difference higher than a chosen threshold, associated to a dark/bright transition. This feature permits to consider, at the same time, two separate measurements so that also the swelling progression of the polymer film can be registered, in addition to the deflection of the cantilever tip. The dilation data are collected by monitoring the change in the thickness of a film supported on a rigid substrate that does not bend during the experiment. To avoid wrong positioning of the sample in the chamber, the micrometer is fixed on two manual roto-translational optical stages, one for the horizontal alignment of transmitter and receiver with the sample, and one devoted to guarantee that the cantilever and the light beam are mutually orthogonal. The temperature is controlled in two stages: first the experimental cell is surrounded by two heating tapes that can reach temperatures up to 200 C, through which the temperature of the bending cantilever equipment is actually controlled; in addition, all the instrumentation is inserted in a thermostatic hood to eliminate the fluctuations of room temperature, due to the day/night cycles, that affect the response of the electronic devices. All the connections are made by stainless steel pipes and valves that are heated with a thermo resistance that can withstand 60 C. The system can operate under vacuum and up to a pressure of 8 bar that represents the working limit for the borosilicate windows of the sample cell. Aluminum as well as stainless steel cantilevers (50.0x10.0x0.275 mm and 50.0x10.0x0.5 mm respectively) were used for the measurement of the deflection, while for the swelling data a stainless steel cylindrical support (about 15 mm in diameter) was used for more reliable measurements. Indeed such an axial-symmetric support allows to overcome the uncertainties that are associated to planar supports when the laser beam is not perfectly parallel to the surface of the cantilever, due to bending or not perfect alignment.
The instrument can work between -40 and +200 C with polymers, polymer melts, coatings, lubricants, food and drinks.